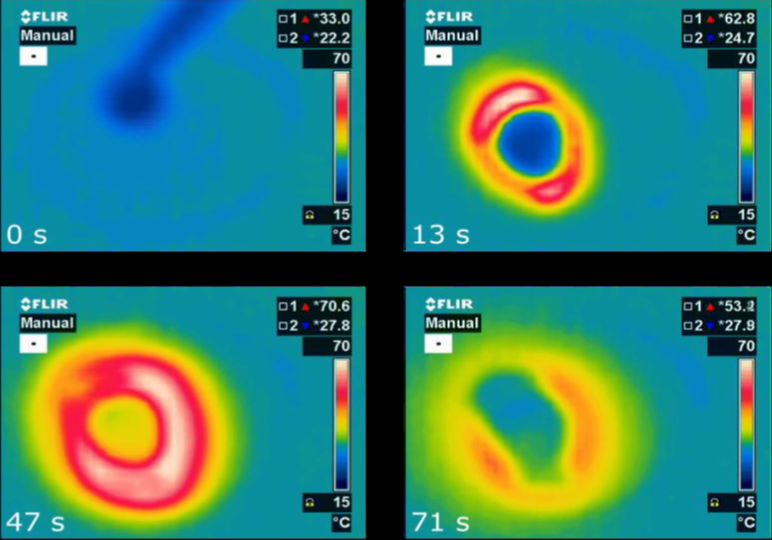
Source: National Institute of Chemistry, Slovenia
Microporous hydrophilic substances such as zeolites are recognised as promising materials for very efficient energy storage. Solar thermal systems are used to dry the material and store all of the thermal energy inside without losses until water is returned into the structure to release sensible heat. An international group of researchers within the IEA Solar Heating and Cooling Programme (IEA SHC) has now discovered an alternative, namely microporous aluminophosphates such as AIPO4-LTA, with properties superior to those of zeolites (link to ). As shown by the charts, a drop of water on 200 mg of AIPO4-LTA releases heat in a matter of seconds.
“The small-pore aluminophosphate material AlPO4-LTA is an excellent choice for sorption-based solar energy storage, superior to all other zeolite-like and metal organic framework materials that we have tested so far,” said Dr Alenka Ristic, Research Associate at the Department of Inorganic Chemistry and Technology of the National Institute of Chemistry in Ljubljana, Slovenia. She heads the working group Development and Characterization of Improved Thermochemical Materials in IEA SHC Task 58.
In a science paper written in 2017, Ristic named the requirements that a well-performing sorption-based energy storage material should meet (see reference at the end of this news article):
- High energy storage capacity owing to increased water uptake
- Easy charging at low temperature
- Low cycle degradation due to great hydrothermal stability
Several types of microporous materials have been considered for thermal energy storage, but the majority has failed to meet the above criteria. For example, the uptake of water into microporous amorphous silica gels is too low, while crystalline zeolites usually require regeneration temperatures that are too high.
All three microporous materials are white powders but show differently shaped crystals when put under a scanning electron microscope. Zeolite 4A is a widely used material, which is already manufactured by Slovenian-based company Silkem.
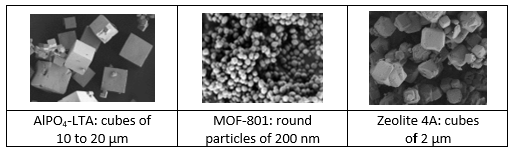
Source: National Institute of Chemistry, Slovenia
The following table shows the increase in storage capacity of aluminophosphates compared to other materials, namely a metal-organic framework (MOF) and a zeolite. In their paper, the scientists list multiple reasons for the improved capacity. For example, the sudden water uptake is achieved by the quick formation of very favourably structured hydrogen-bonded water clusters in the pores of AlPO4. Additionally, the weak adsorption centres facilitate dehydration at lower temperatures.
|
|
Maximum water uptake (g/g)
|
Energy storage capacity (Wh/kg)
|
Charging temperature
(oC)
|
Decrease in water capacity (%) after 20 cycles
|
|
AlPO4-LTA
|
0.42
|
373
|
65
|
< 0.3
|
|
MOF -801
|
0.36
|
323
|
80
|
> 3
|
|
Zeolite 4A
|
0.28
|
350
|
300
|
< 0.3
|
Key features of microporous materials. MOF is short for metal organic framework.
Source: National Institute of Chemistry, Slovenia
Currently, AlPO4-LTA is being synthesised in a laboratory at the National Institute of Chemistry. Ristic said that production without so called structure-directing agents was a great advantage. “Our next step will be to upscale the template-free synthesis of AlPO4-LTA by using cheaper raw reactants because no expensive structure-directing agents are required,” the researcher added. It will depend on the degree of upscaling whether the material can already be used in small heat storage prototypes, for example, inside electric vehicles.
More information:
http://task58.iea-shc.org/
Science paper:
Krajnc, A., Varlec, J., Mazaj, M., Ristic, A., Zabukovec Logar, N., Mali, G. (2017). Superior performance of microporous aluminophosphate with LTA topology in solar-energy storage and heat reallocation. Advanced Energy Materials, 7(11), 1-8. doi: 10.1002/aenm.201601815. attached https://onlinelibrary.wiley.com/doi/full/10.1002/aenm.201601815